Frequently Asked Questions
MD Innovate, Inc.

How are PneumoniaCheck™ and PneumoKazoo™ specimens analyzed?
The PneumoniaCheck™ and PneumoKazoo™ filters can be analyzed by commercial laboratories using a variety of methods. Analysis of the filter may include traditional culture and sensitivity, gram stain, immunoassay, or DNA (PCR) testing.
Have PneumoniaCheck™ and PneumoKazoo™ been tested?
The device has been extensively tested to collect particles in aerosols on a high efficiency filter, exclude oral liquids and solids from the sample, and selectively separate lung aerosols. These validation tests have been peer-reviewed and published in the Journal of Medical Devices.
Are PneumoniaCheck™ and PneumoKazoo™ patented?
Yes, PneumoniaCheck™ and PneumoKazoo™ are patented. Both are manufactured under license from Georgia Tech Research Corporation and the US Centers for Disease Control and Prevention, USA.
How does PneumoniaCheck™ and PneumoKazoo™ work?
PneumoniaCheck™ and PneumoKazoo™ both use fluid mechanics to separate the liquid and solid contaminants in your mouth from the aerosols in your lungs.

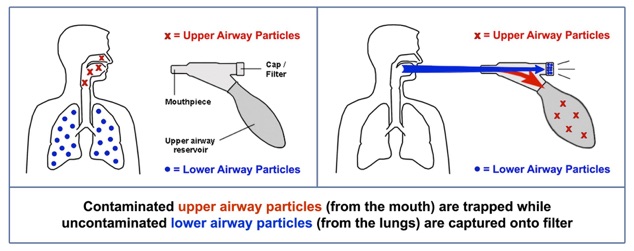
How does PneumoniaCheck™ and PneumoKazoo™ collect pathogens from the lungs?
PneumoniaCheck™ and PneumoKazoo™ both utilize a medical grade filter that has been tested and shown to capture over 99% of bacteria and viruses.
The Bacterial Filtration Efficiency and Viral Filtration Efficiency ratings are >99.939% and >99.856%, respectively.
Are PneumoniaCheck™ and PneumoKazoo™ approved by the FDA?
PneumoniaCheck™ and PneumoKazoo™ are cleared for sale by the FDA in the United States.
MD Innovate, Inc. | 404.585.8109 | info@MDinnov8.com | 201 W Ponce de Leon Ave | Suite 317 | Decatur, Georgia 30030
